(MEDICAL DEVICES)
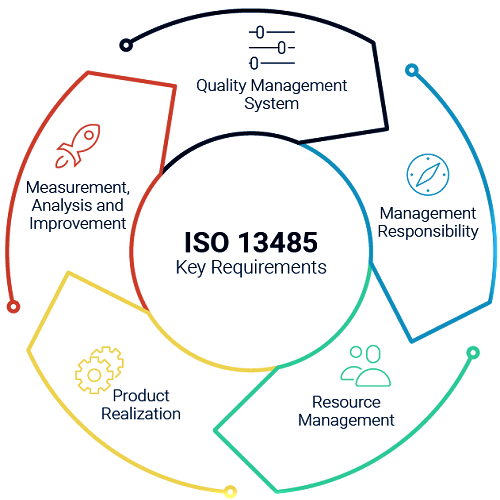
Joining of hazard-based methodologies past item acknowledgment. Therefor, Hazard is considered with regards to the security and execution of the clinical gadget. And, in addressing administrative requirements;
Improved linkage with administrative requirements, especially for administrative documentation.
Being, Application to associations all through the lifecycle and gracefully chain for clinical gadgets.
Therefore, Harmonization of the requirements for programming approval for various programming applications (QMS programming, system control programming, programming for checking and estimation) in various provisos of the norm;
Accentuation on the right framework, especially for the creation of clean clinical gadgets, and expansion of requirements for approval of sterile boundary properties
So, Extra needs in structure and improvement on the thought of ease of use, utilization of gauges, confirmation and approval arranging, plan move and configuration records;
Accentuation on protest taking care of and answering to administrative experts as per administrative necessities, and thought of post-showcase observation;
Arranging and recording restorative activity and preventive activity, and applying remedial activity without undue deferment. BMS provide certification of ISO 13485 in Karachi Pakistan.
Talk to us now to book in one of our experts